usp class vi pdf
When evaluating a new product many of our. USP Class IV USP Class V USP Class VI USP Class IV USP Class VI USP Class VI USP Class IV USP Class VI USP Class VI.

Usp Y W Technologies Finish With The Best
Consumers implicitly rely upon the standards put into place by governing agencies to protect the publics health and well-being.

. Pharmacopeial Conven-tion USP is a non-commercial organisation that develops the standards for the quality of medications. L929 MEM Elution Test USP S60121 Article meets the requirements of the test and is not considered to have a cytotoxic effect. USP Class VI ISO 10993-5 Cytotoxicity In-Vitro ISO 10993-3 Ames Genotoxicity ISO 10993-11 Systemic Toxicity In-Vivo ISO 10993-4 Hemolysis Indirect European Pharmacopeia 329.
USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and vegetable oil and injecting it in specimen rabbits and. Our USP Class VI certified material offering includes. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a desired function without causing adverse effects on the.
USP Class VI Permanent USP Class V Prolonged USP Class IV Limited Blood Path Indirect Mucosal Surfaces Limited USP Class I USP Class III Permanent USP Class V. Time tested for USP Class VI compliance This article was manufactured using procedures typically required to produce final parts. Pharmacopeia USP group is a non-government non-profit organization that sets the standards for the production of drugs both human and.
Take an ASTM D2000 call out. Typically the terms USP Class VI or ISO 10993 materials are used. Guidelines of USP Class VI pharmaceutical approval.
USP Class VI compliant O-Rings N ewmans USP Class VI compliant Platinum Cured Silicone O-Rings are complimented with our Class VI EPDM black or whiteperoxide cured and Viton. The sample is designated as E553 Article within our. Unlike other rubber standards theres no one standard that engineers use for an approval.
Learn about USPs portfolio of solutions to help address quality assurance enhance regulatory predictability and help manufacturers distribute quality medicines dietary supplements and. SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. Moldable polyurethanes Resilon 4300 and 4301 Molythane 4615 Machinable polymer-filled 0618 PTFE Life Sciences Capabilites.
SIMONA PP-H USP Class VI sheet material is easy to clean. USP Class VI compliant. Biological Test for Plastics USP Class VI 121oC S60121.
When the surface area of the the class designation of a plastic must be accompanied by specimen cannot be determined use 01 g of elastomer oran indication of the temperature of.
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants
Flanged Clamp Gaskets Newman Sanitary Gasket Company Inc

What Is Usp Class Vi Testing Tbl Plastics

Silcon Medical Grade Silicone Tubing Newage Industries

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe

Gauge Guard Protector Literature Resources Rubber Fab

Usp Class Vi Testing Aft Fluorotec

Data Sheets Panacol Elosol Gmbh

Keofitt World Leaders In Sterile Sampling

Printing Equipment For The Medical Device Industry Printing International Pdf Catalogs Technical Documentation Brochure
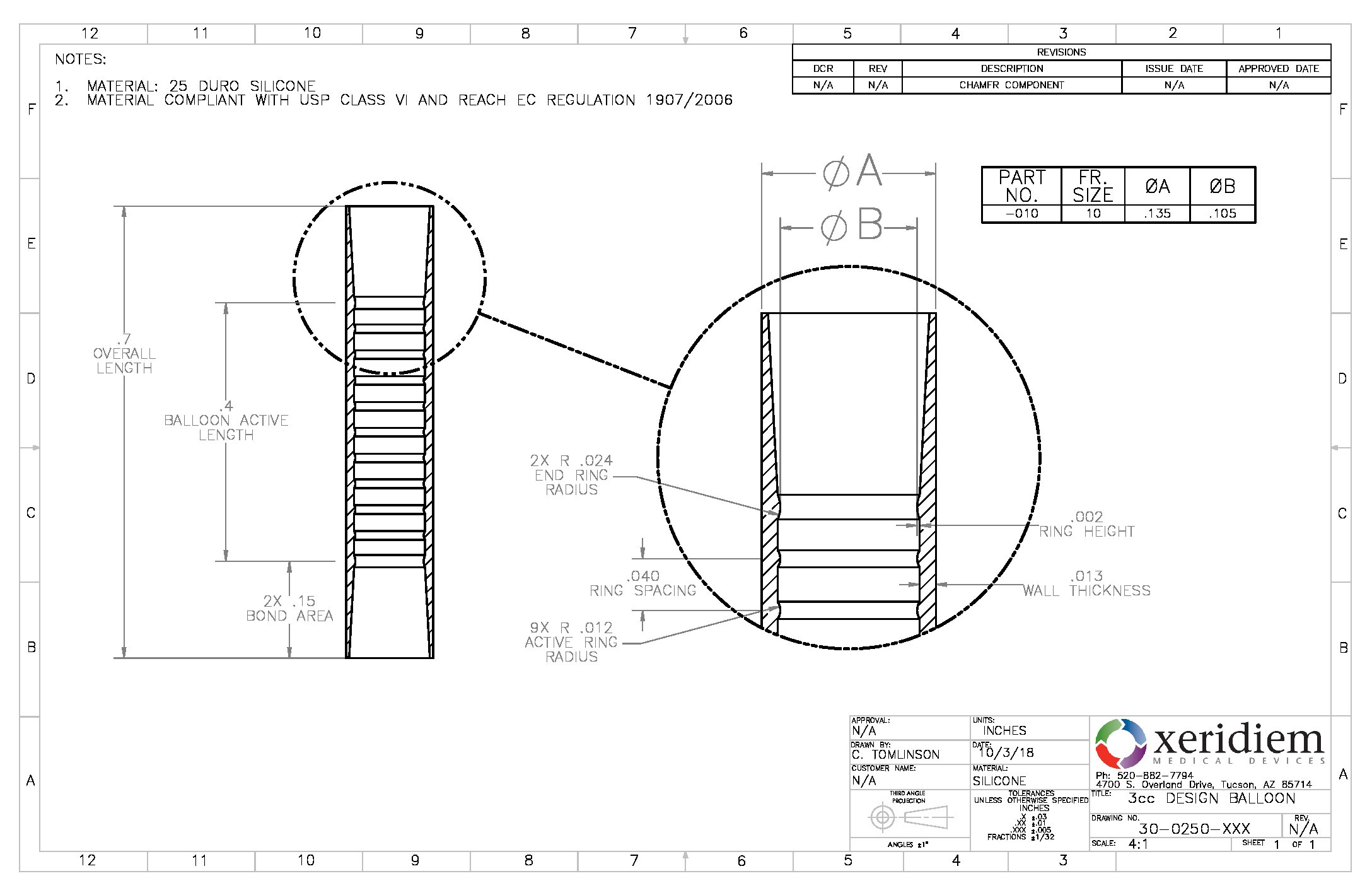
Balloon Silicone 3 4mm Od Spherical Quantity 10 Bag

Pdf Brochure And Data Sheet On The Pure Fit Spt 50 Sti

Heat Shrink Fep Tubing Mt Fep Altera Medical Grade Usp Class Vi

Elast O Purea Hygienic Clamp Gaskets James Walker

Hp Jet Fusion 3d Printing Aerosport Additive

Stc The Value Of Usp Class Vi Testing For Plastics Used In Medical Devices Stc

Sanitrx Hpx Ii Continental Disc Corp Pdf Catalogs Technical Documentation Brochure

